
L-DNA
L-DNA
L-DNA
(Attention: not to be confused with LNA=Locked Nucleic Acid)
In the case of nucleic acids, nature used only one possible stereoisomer of ribose or deoxyribose, namely, the D-form. Double strands of these natural nucleic acids form a right-handed helix.
From the other enantiomer of this sugar, L-deoxyribose, one can chemically synthesised appropriate monomers and then oligonucleotides, so-called L-DNA. Two complementary strands of this not naturally occurring L-form form a left handed helix. The stability of the L-DNA is comparable to the corresponding D-DNA duplexes and behave like mirror images to this. Mixed duplexes of L-DNA and D-DNA strand do not exist. L-DNA is relatively stable against enzymatic degradation.
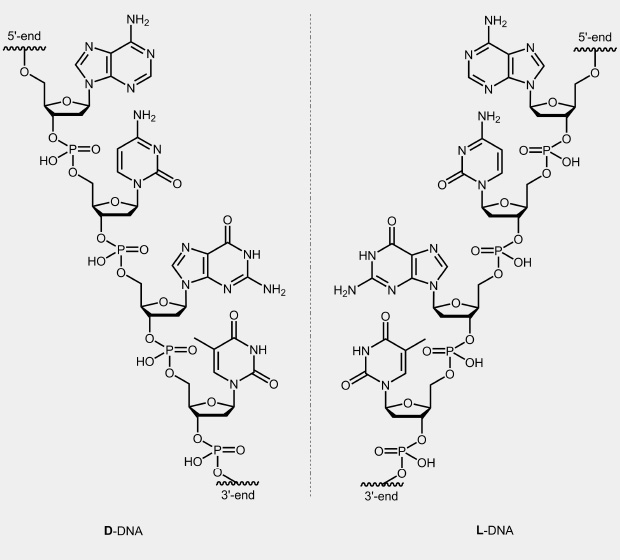 |
biomers.net offers the synthesis of oligonucleotides exclusively build with L-DNA (additionally modified with dyes) as well as D-DNA / L-DNA chimeras.
Literature:
- Superior Structure Stability and Selectivity of Hairpin Nucleic Acid Probes with an L-DNA Stem. Kim Y, Yang CJ, Tan W; Nucleic Acids Research (2007), 35(21): 7279-7287.
- Utilising the left-helical conformation of L-DNA for analysing different marker types on a single universal microarray platform. Hauser NC, Martinez R, Jacob A, Rupp S, Hoheisel JD, Matysiak S; Nucleic Acids Research (2006), 34, 5101-5111.
- Application of L-DNA as a molecular tag. Hayashi G, Hagihara M, Nakatani K; Nucleic Acids Symp Ser (2005), 49, 261-262.

