
Chelate complex modifications
DTPA
DTPA modified oligonucleotides
Diethylenetriaminepentaacetic acid (DTPA), also known as pentetic acid, is a chelating agent and capable of forming extremely stable complexes with metal salts. DTPA is an aminopolycarboxylic acid composed of a diethylenetriamine backbone with five carboxymethyl groups.
DTPA has a strong affinity for metal cations with large ionic radius. In addition to various heavy metal ions, the slightly smaller iron ions can also be bound efficiently.
Assuming that each nitrogen atom and carboxyl group can act as a potential coordination partner, the pentaanion DTPA5- has up to eight free binding sites. Here, DTPA literally wraps around the metal ion. Since some complexes with certain metals require fewer coordination sites, DTPA can bind additional reaction partners even after complexation of metal ions.
Chelates, consisting of gadolinium ions (Gd3+) and DTPA, form extremely strong complexes that are also used intracellularly. For example, complexed Gd ions are used as contrast agents in magnetic resonance imaging (MRI) to enhance contrast imaging.
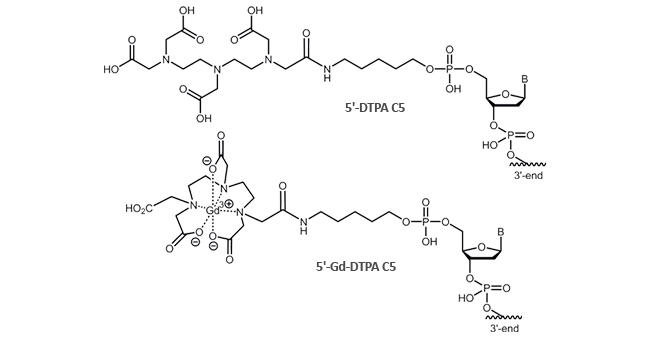
DTPA can be attached to the 5´-terminus of the oligonucleotide. The modification is possible up to a length of
45 bases.
Literature:
1. Gadolinium(III) Complexes with N-Alkyl-N-methylglucamine Surfactants Incorporated into Liposomes as Potential MRI Contrast Agents. Silva RS, Correia Duarte E, Ramos GS, Kock FVC, Diuk Andrade F, Frézard F, Colnago LA, Demicheli C; Bioinorganic Chemistry and Applications (2015), Volume 2015, Article ID 942147, 8 pages.
2. Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent. Weinmann HJ, Brasch RC, Press WR, Wesbey GE. AJR Am J Roentgenol. (1984); 142(3):619-24.
3. Gadolinium-DTPA as a contrast agent in MRI: initial clinical experience in 20 patients. Carr DH, Brown J, Bydder GM, Steiner RE, Weinmann HJ, Speck U, Hall AS, Young IR; American Journal of Roentgenology. (1984); 143: 215-224. 10.2214/ajr.143.2.215.
4. Molecular MR Contrast Agents for the Detection of Cancer: Past and Present. Bogdanov A, Mazzanti ML; Semin Oncol. (2011); 38(1): 42–54.
5. Macromolecular MRI contrast agents: Structures, properties and applications. Tang J, Sheng Y, Hu H, Shen Y; Progress in Polymer Science (2013); Volume 38, Issues 3–4, Pages 462-502.
DOTA
DOTA modified oligonucleotides
DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) contains four acetic acid carboxylate groups on the four nitrogen atoms of its twelve-membered cyclic structure. Similar to DTPA, DOTA is also capable of binding divalent and trivalent metal ions (e.g. gadolinium Gd3+) to form stable chelate complexes. Additionally, after complexation of a metal ion DOTA can also covalently bind proteins and other biomolecules (antibodies, peptides, biotin, etc.) via one of the up to eight binding sites.
Due to its high in vivo and in vitro stability DOTA is used in combination with complexed Gd-ions as a chelating complexing agent in the contrast medium in magnetic resonance imaging (MRI).
The chelator DOTA is available as a 5´-modification to the oligonucleotide. DOTA is bound to the oligo using the click-reactive DBCO.
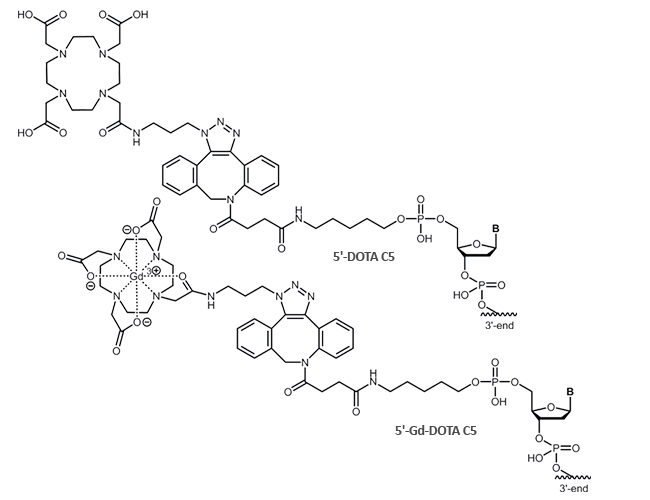
Literature:
1. Labeling of Oligonucleotides with DTPA and DOTA on Solid Phase. Hovinen J; Nucleosides, Nucleotides and Nucleic Acids (2007), Volume 26, Issue 10-12.
2. Solid-phase oligonucleotide labeling with DOTA. Jaakkola L, Ylikoski A, Hovinen J; Curr Protoc Nucleic Acid Chem. (2007), Chapter 14:Unit 4.31. doi: 10.1002/0471142700.nc0431s29.
3. Isomerism in benzyl-DOTA derived bifunctional chelators: implications for molecular imaging. Payne KM, Woods M; Bioconjug Chem. (2015), 26(2):338-44. doi: 10.1021/bc500593h.
4. Synthesis of a gadolinium basedmacrocyclic MRI contrast agent for effective cancer diagnosis. Jeong Y, Na K; Biomaterials Research (2018) 22:17, doi.org/10.1186/s40824-018-0127-9.
5. Pre-targeted radioimmunotherapy of human colon cancer xenografts in athymic mice using streptavidin-CC49 monoclonal antibody and 90Y-DOTA-biotin. Domingo RJ, Reilly RM; Nuclear Medicine Communications (2000), 21(1):89-96.
NTA
NTA oligo conjugates
Another representative of the chelating agents is nitrilotriacetic acid (NTA). After complexing divalent metal ions (Ni2+, Cu2+, etc.), NTA has a high affinity for polyhistidine-labelled molecules (e.g. recombinant proteins) and binds them very efficiently. Especially in the field of purification and isolation of recombinant proteins (metal affinity chromatography), this property of the chelating agent NTA is already known.
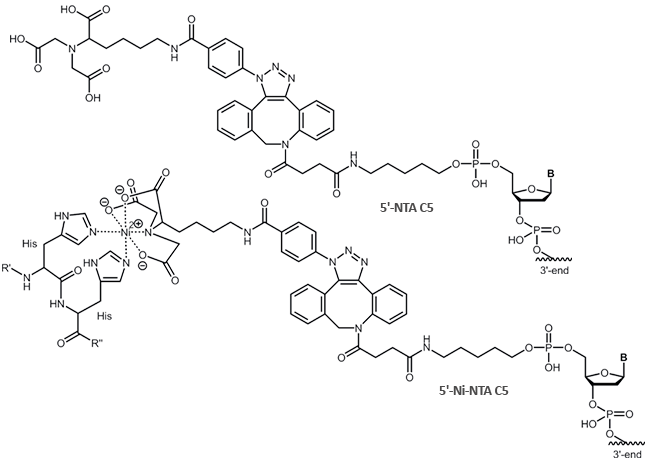
Coupled to a DNA oligonucleotide, the property of His-tag binding can be exploited to form, for example, DNA-protein conjugates.
NTA is linked to an oligo (5´- or 3'-terminus) using an azide via the copper free click chemistry.
Literature:
1. Conjugation of DNA with protein using His-tag chemistry and its application to the aptamer-based detection system. Shimada J, Maruyama T, Hosogi T, Tominaga J, Kamiya N, Goto M; Biotechnology Letters (2008), Volume 30, Issue 11, pp 2001–2006.
2. Oligohis-tags: mechanisms of binding to Ni2+-NTA surfaces. Knecht S, Ricklin D, Eberle AN, Ernst B; J Mol Recognit. (2009); 22(4):270-9. doi: 10.1002/jmr.941.
3. DNA-enzyme conjugate with a weak inhibitor that can specifically detect thrombin in a homogeneous medium. Shimada J, Maruyama T, Kitaoka M, Kamiya N, Goto M; Anal Biochem. (2011), 414(1):103-8. doi: 10.1016/j.ab.2011.02.035.

